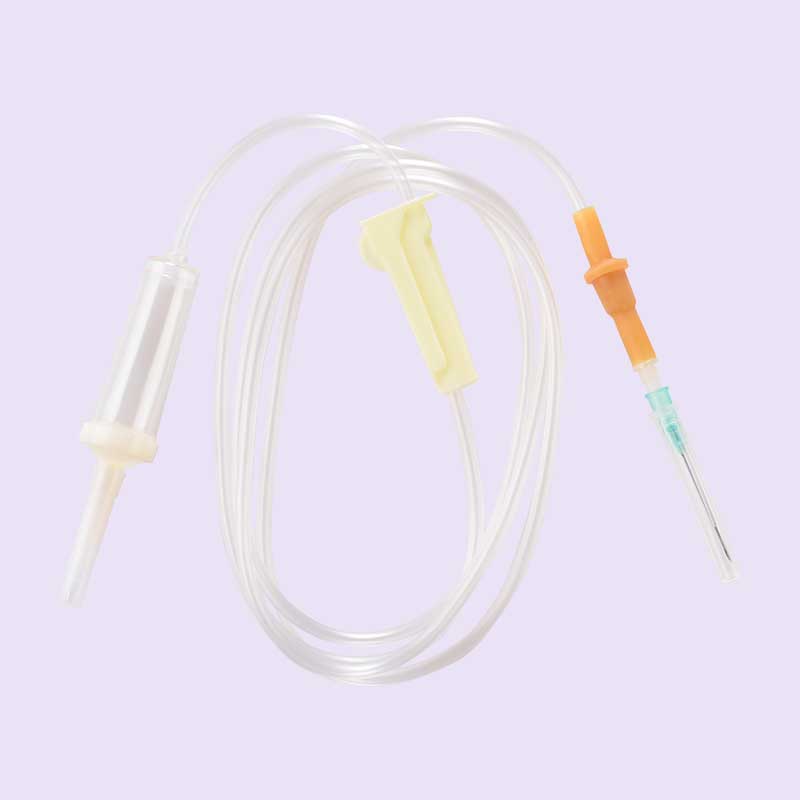
Brief Description
The Intravenous sets are utilized for infusion of intravenous body fluids contain following components to make it complete and also to fulfill its requirements of intended use in compliance of ISO 8536:4;
- Spike Cover : The protective cap is manufactured from medical grade LDPE to offer due protection of Closure Piercing Device/Spike and better penetration of EO for sterilization
- Closure Piercing Device/Spike :Made frommedical grade plastic (ABS) material to offer superiorperformance and available in below detailed variants;
- Non Vented : To connect the fluid container to administer the fluid in to the device by piercing the container with the help of spike tip. To provide air ventilation for infusion of fluid an appropriate size of hypodermic needle to be used to puncture the fluid
- Vented : A provision of filtered air inlet /vent filter is provided with closure piercing device to facilitate the device for entry of filtered air to help for infusing the
- Drip Tube:
- Fluid Volume (60Drops/ml): To facilitate the device for drop formation in a very precise way, a stainless steel made tube inserted in its housing to be assembled with closure piercing device.
- Fluid Volume (20Drops/min): Provision of molded drip tube to facilitate the device for drop formation
- Drip Chamber: To collect the intravenous fluid for further discharge in a smooth manner. The transparency of the chamber facilitates the easy monitoring of drop fall. It is made of virgin grade, non-toxic, non irritant PVC material
- Fluid Filter: Provided for filtration of unwanted material present in I.V. fluids.
- PVC Tubing: Made of virgin grade, non-toxic, non irritant PVC material to provide sufficient path for connection of device with the patient.
- Flow Regulator: Made from medical grade plastic (ABS) material to offer superior performance to adjust the flow of the infusion solution between Zero and the maximum smoothly to comply the requirements of ISO 8536-14:2016.
-
Injection site: To administer extra medication with the fluid as per requirement. The injection site contains self sealing property to avoid leakage of fluid.
- End Connector/ Adopter: The 6% luer taper of the component facilitates the leak proof joint with the Hypodermic Needle or any other combination of medical device in compliance of ISO 80369-7:2016.
- Hypodermic Needle: The stainless steel made hypodermic needle having appropriate bevel angle facilitates easy insertion of the same in to the venous system of the patient.
- Needle Cover: To protect the tip of Hypodermic Needle
INTENDED USE: The Intra Venous sets also known as I.V. giving sets are intended for use alone or in combination with other medical devices to deliver life-saving medications like antibiotics, fluids required to cure the infectious diseases, saline, glucose or any other desirable fluids contained in an fluid container in to the patient’s venous system to treat the same by utilizing gravitational administration technique.
KEY FEATURES:
- Sharp strong spike for smooth penetration into bottle/container.
- Transparent and flexible drip chamber facilities visual access and for rapid adjustment.
- Transparent chamber for easy visualization of flow.
- Connecting ports with 6% taper & luer lock facility.
- High quality kink-resistant tubing with smooth inner surfaces for non-turbulent flow.
- Designed as per 20drops are equivalent to 1 ± 0.1 ml
- Transparent individual packed for visibility of complete product
- Transparent & Flexible tubing
- Kink resistant tubing
- Self-sealing latex bulb for extra medication and easy flushing
Specifications : TUBE LENGTH – 1500 mm (±5 mm)
Available Variants: VENTED – NON-VENTED
Available Variants: VENTED – NON-VENTED
